Abstract
Background: High-dose melphalan (Mel) 200 mg/m2 is considered the standard of care preparative regimen for autologous hematopoietic stem cell transplantation (auto-HCT) for multiple myeloma. Two recent retrospective analyses suggested that a combination of busulfan (Bu) and Mel (Bu-Mel) may be associated with a longer progression-free survival (PFS) compared to Mel alone. In this randomized phase III trial we compared the safety and efficacy of Bu-Mel vs. Mel.
Methods: Patients were randomized to either Bu-Mel or Mel using the Pocock-Simon method within 12 months of the start of induction therapy. In the Bu-Mel arm, Bu 130 mg/m2 was infused daily for 4 days, either as a fixed dose or to target an average daily area under the curve of 5000 μmol-min, followed by 2 daily doses of Mel at 70 mg/m2. Mel 200 mg/m2 was given as a single dose in the Mel only arm. The trial was designed to detect a 14-month increase in median PFS in the Bu-Mel arm with two-sided tests having nominal overall type I error .029 and power .80, with up to 3 tests using O'Brien-Fleming decision boundaries. The primary objective was to compare PFS between the two arms. Secondary objectives were to compare the response rates, toxicity and overall survival (OS).
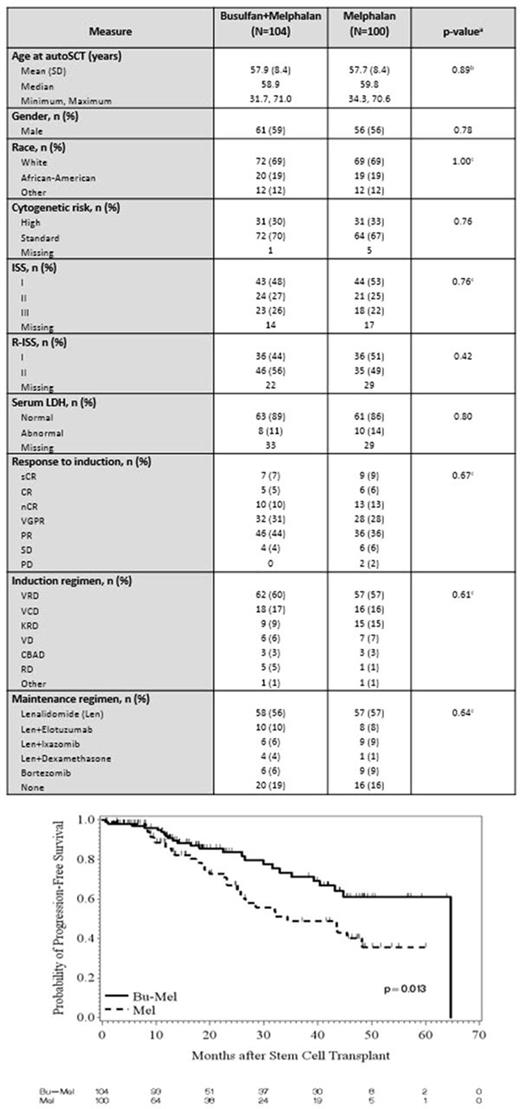
Results: Two-hundred and four patients (Bu-Mel: 104, Mel: 100) were enrolled from October 2011 to March 2017. Patient characteristics are summarized in Table 1. There was no significant difference between the two arms in age, gender, race, cytogenetic risk status, ISS stage, serum LDH, induction regimens, response to induction, or maintenance therapy. One-hundred-day non-relapse mortality (NRM) was 0% in both arms and 1-year NRM was 2% vs. 0% in Bu-Mel vs. Mel (p=0.11). NCICTCv3.0 grade 1-3 diarrhea was seen in 59 (57%) and 78 (78%) patients in Bu-Mel vs. Mel, respectively (p=0.002). Grade 1-3 mucositis (96% vs. 49%, p <0.001), rise in ALT (33% vs 1%, p <0.001) and neutropenic fever (71% vs. 30%, p <0.001) were seen at a higher rate in the Bu-Mel arm. No second primary malignancies have been seen in either arm to date. At day 90 after auto-HCT, 27 (26%) and 33 (33%) patients had achieved complete remission (CR; p=0.29), and 49 (47%) and 55 (55%) patients had achieved at least near CR (nCR; p=0.27) in Bu-Mel and Mel, respectively. Minimal residual disease (MRD) analysis by multiparametric flow cytometry (MFC) was performed at day 100 in 86 and 77 patients in Bu-Mel and Mel, respectively. Fifty (58%) and 47 (61%) achieved MRD negative status in Bu-Mel and Mel arms, respectively (p=0.75). At last evaluation, 52 (51%) and 49 (49%) patients had achieved a CR (p=0.89), and 70 (67%) and 67 (67%) patients had achieved at least nCR (p=1.00) in Bu-Mel and Mel, respectively. Median follow up was 22.1 months (range 0.7 - 64.8). Kaplan-Meier estimates of median PFS was 64.7 months in the Bu-Mel arm, and 34.4 months in the Mel only arm (p=0.013) (Figure 1). This significant difference in PFS was maintained after adjusting for the type of maintenance therapy received (p=0.019). With a median follow up of 28.1 months, there was no difference in OS between the Bu-Mel or Mel only arms (p=0.94). In a fitted Bayesian regression model, the posterior probability that patients receiving Bu-Mel had a smaller risk of progression and/or death compared with patients receiving Mel alone is 0.982, which is highly significant.
Conclusions: In this phase III trial, Bu-Mel regimen was safe, and associated with a significantly longer PFS than Mel alone. This significant difference in PFS continued even after adjusting for maintenance therapy. Potential explanations for a longer PFS without a significant difference in response rates include a deeper MRD negativity or selective targeting of clonogenic myeloma progenitor cells by Bu-Mel.
Shah: Indapta Therapeutics: Other: Stock Ownership; Celgene: Research Funding; Celgene, Indapta Therapeutics, Takeda: Consultancy; TeneoBio, Inc.: Honoraria. Patel: Juno: Consultancy; Celgene: Consultancy; Pfizer: Membership on an entity's Board of Directors or advisory committees, Research Funding. Thomas: Celgene: Research Funding; Bristol Myers Squibb: Research Funding. Manasanch: celgene: Consultancy; quest diagnostics: Research Funding; adaptive biotechnologies: Consultancy; sanofi: Research Funding; takeda: Consultancy; merck: Research Funding. Lee: Adaptive: Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy; Eutropics Pharmaceuticals: Research Funding; Daiichi Sankyo: Research Funding; Pimera Inc: Consultancy; Takeda: Consultancy. Orlowski: BioTheryX: Consultancy, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal